trigonal bipyramidal|trigonal bipyramidal vs trigonal pyramidal : Manila The VSEPR theory also predicts that substitution of a ligand at a central atom by a lone pair of valence electrons leaves the general form of the . See more Resultado da Start the game Mirax 5.0 rating LTC 5.0 rating TonyBet 5.0 rating Hell Spin 5.0 rating 21Bit 5.0 rating PartyCasino 4.0 rating Gioo 5.0 rating Golden Star 5.0 rating Rolling Slots 5.0 rating
0 · trigonal planar vs trigonal pyramidal
1 · trigonal bipyramidal vs trigonal pyramidal
2 · trigonal bipyramidal vs pyramidal
3 · trigonal bipyramidal polar or nonpolar
4 · trigonal bipyramidal axial vs equatorial
5 · trigonal bipyramidal 3 lone pairs
6 · how to draw trigonal bipyramidal
7 · can trigonal bipyramidal be nonpolar
8 · More
Tell Me a Story (1ª Temporada) avaliado por quem mais entende de séries, o público. Faça parte do Filmow e avalie esta série você também. Login Cadastre-se Início Filmes .
trigonal bipyramidal*******In chemistry, a trigonal bipyramid formation is a molecular geometry with one atom at the center and 5 more atoms at the corners of a triangular bipyramid. This is one geometry for which the bond angles surrounding the central atom are not identical (see also pentagonal bipyramid), because there is no . See moretrigonal bipyramidal trigonal bipyramidal vs trigonal pyramidalThe five atoms bonded to the central atom are not all equivalent, and two different types of position are defined. For phosphorus pentachloride as . See moreThe VSEPR theory also predicts that substitution of a ligand at a central atom by a lone pair of valence electrons leaves the general form of the . See moreIsomers with a trigonal bipyramidal geometry are able to interconvert through a process known as Berry pseudorotation. Pseudorotation is similar in concept to the movement of a conformational diastereomer, though no full revolutions are completed. In . See more• AXE method• Molecular geometry See more
• Indiana University Molecular Structure Center• Interactive molecular examples for point groups• Molecular Modeling See more Trigonal Bipyramidal Molecular Geometry. NOTES: This molecule is made up of 5 sp 3 d hybrid orbitals. Three orbitals are arranged around the equator of the .It is a trigonal bipyramid with three missing equatorial vertices. A The tin atom donates 4 valence electrons and each chlorine atom donates 7 valence electrons. With 18 valence .
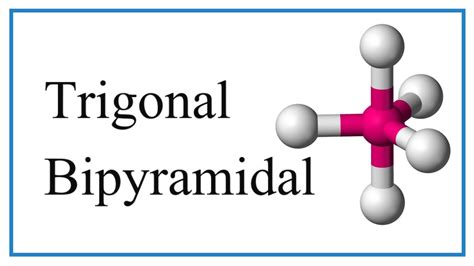
Molecular Geometry of the Trigonal Bipyramidal Structures. The order of most repulsion to least repulsion among bonding and lone pair electrons are: To decide . In this video we’ll look at the Trigonal Bipyramidal Molecular Geometry and Bond Angles. We'll use the example of PCl5 to understand the molecular shape. . Learn about trigonal bipyramidal molecular geometry, which has a central atom with five bonds and two different bond angles. See examples of molecules . Learn how to apply VSEPR theory to molecules and ions with five electron clouds around the central atom. See examples of trigonal bipyramidal and seesaw geometries, and how lone pairs affect bond angles.This web page is part of a free textbook on chemistry that covers molecular structure and polarity. However, the web page has a glitch and cannot display the content related .trigonal bipyramidal vs trigonal pyramidalTrigonal Bipyramidal Geometry. Trigonal bipyramidal arrangement of 5 regions of high electron density (white). Three regions of high electron density point at the corners of an .Learn about the molecular geometry and polarity of molecules with trigonal bipyramidal electronic geometry, such as SF4, IF3, and XeF2. See how lone pairs .Trigonal Bipyramidal Electronic Geometry: AB 5, AB 4U, AB 3U2, and AB 2U 3 |If lone pairs are incorporated into the trigonal bipyramidal structure, there are three possible new shapes. 1. One lone pair - Seesaw shape 2. Two lone pairs - T-shape 3. Three lone pairs – linear |The lone pairs occupy equatorial positions becauseAll molecules with 5 electron domains have trigonal bipyramidial electronic geometry. The central atom of these molecules must be in the third or higher period of the periodic table. Figure \(\PageIndex{7}\): trigonal .
Triangular bipyramid. In geometry, the triangular bipyramid is the hexahedron with six triangular faces, constructed by attaching two tetrahedra face-to-face. The same shape is also called the triangular dipyramid [1] [2] or trigonal bipyramid. [3] If these tetrahedra are regular, all faces of triangular bipyramid are equilateral.The base angles are still 180°, 120°, and 90° while the tweaked angle will now be slightly less in each case due to the extra repulsion from the lone pair. POLARITY: POLAR - The lone pair electrons throw off the perfectly cancelling symmetry of the five trigonal bipyramidal regions thus making the overall molecule polar.
In this video, we apply VSEPR theory to molecules and ions with five groups or “clouds” of electrons around the central atom. To minimize repulsions, five electron clouds will always adopt a .
A trigonal bipyramidal shape forms when a central atom is surrounded by five atoms in a molecule. In the geometry, three atoms are in the same plane with bond angles of 120°; the other two atoms are on opposite ends of the molecule. Some elements in Group 15 of the periodic table form compounds of the type [latex]\text{AX}_5[/latex]; examples .
The bond angle can help differentiate between linear, trigonal planar, tetraheral, trigonal-bipyramidal, and octahedral. The ideal bond angles are the angles that demonstrate the maximum angle where it would minimize repulsion, thus verifying the VSEPR theory. Essentially, bond angles is telling us that electrons don't like to be near each other.

Total electrons = 28. An octet of electrons exists in every fluorine atom, and an expanded octet exists in every chlorine atom. Trigonal bipyramid is the electron pair geometry, and T-shape is the molecular geometry. The axial atoms are slightly bent from their original 180-degree angle once more. BH3, a three-atom compound with no lone pair.A trigonal bipyramidal molecule is a little more difficult because there are more atoms and bonds to consider. A trigonal bipyramidal form resembles two three-sided pyramids sharing a side, as the name suggests. It has a steric number of 5 because it has a core atom with five bonds. The trigonal bipyramidal is made of hybridization.
Trigonal Bipyramidal. The Trigonal Bipyramidal is a molecular shape where there are 5 bonds attached to a central atom. There are two bond angles for this shape. The first one is 90 degrees and the second one is 120 degrees. The shape is non-polar since it is symmetrical. There are no lone pairs attached to the central atom. The bond angle can help differentiate between linear, trigonal planar, tetraheral, trigonal-bipyramidal, and octahedral. The ideal bond angles are the angles that demonstrate the maximum angle where it would minimize repulsion, thus verifying the VSEPR theory. Essentially, bond angles is telling us that electrons don't like to be near . To see all my Chemistry videos, check outhttp://socratic.org/chemistryIf the central atom in a molecular can make 5 bonds, the structure that it makes is bas.Trigonal pyramidal molecular geometry. In chemistry, a trigonal pyramid is a molecular geometry with one atom at the apex and three atoms at the corners of a trigonal base, resembling a tetrahedron (not to be confused with the tetrahedral geometry ). When all three atoms at the corners are identical, the molecule belongs to point group C3v.The T-shaped geometry is related to the trigonal bipyramidal molecular geometry for AX 5 molecules with three equatorial and two axial ligands. In an AX 3 E 2 molecule, the two lone pairs occupy two equatorial positions, and the three ligand atoms occupy the two axial positions as well as one equatorial position. The three atoms bond at 90 .In chemistry, a trigonal bipyramid formation is a molecular geometry with one atom at the center and 5 more atoms at the corners of a triangular bipyramid.The molecular geometry of PCl 5 is trigonal bipyramidal, as shown in Figure \(\PageIndex{3}\). The molecule has three atoms in a plane in equatorial positions and two atoms above and below the plane in axial positions.trigonal bipyramidal Trigonal Bipyramidal structures are electronic configurations of molecules. VSEPR gives us a handful of parent shapes. The one with 5 sets of bonded or non-bonded electrons.
Three orbitals are arranged around the equator of the molecule with bond angles of 120 o. Two orbitals are arranged along the vertical axis at 90 o from the equatorial orbitals. The shape of the orbitals is trigonal bipyramidal.In this video we’ll look at the Trigonal Bipyramidal Molecular Geometry and Bond Angles. We'll use the example of PCl5 to understand the molecular shape. .
WEBwww.redecanais.vg
trigonal bipyramidal|trigonal bipyramidal vs trigonal pyramidal